
A Pulpdent é uma empresa familiar de investigação e fabrico de produtos dentários, fundada em 1947 e empenhada nos seus princípios fundadores de educação, prevenção e cuidados dentários pró-activos, para que as pessoas possam viver vidas mais saudáveis e produtivas.
A investigação e o desenvolvimento de produtos da Pulpdent visam libertar os poderes curativos da natureza com materiais bioactivos que imitam as propriedades físicas e químicas da estrutura dentária, comportam-se favoravelmente no ambiente oral húmido e maximizam o potencial de remineralização.


Princípios fundadores
O fundador da Pulpdent, Dr. Harold Berk, exerceu medicina dentária durante quase 65 anos e foi professor na Faculdade de Medicina Dentária da Universidade Tufts de 1946 a 2005. Os princípios fundamentais do Dr. Berk continuam a orientar a empresa:
Os produtos Pulpdent são fabricados em Watertown, Massachusetts, EUA, em conformidade com o Sistema de Qualidade da empresa e todos os requisitos regulamentares. Todos os anos renovamos o nosso compromisso de investir na investigação e em novas tecnologias, ganhando a confiança da profissão, inspirando os médicos com novas ideias e materiais, e fornecendo a liderança para atingir estes objectivos.
Distribuição mundial
PulpdentO portfólio de produtos em expansão da empresa é distribuído globalmente através de parceiros de distribuição locais. O seu crescimento permitiu-lhe expandir o alcance dos seus produtos únicos que fazem avançar o padrão de cuidados.
Prémios e reconhecimentos
Quase um século de história
PulpdentA história da SMART™ está ancorada num compromisso com a investigação dentária original. Ao longo dos anos, esta investigação conduziu a tecnologias químicas patenteadas e a materiais dentários SMART™ que melhoram ativamente a saúde oral e alteram o paradigma da indústria relativamente aos padrões dos materiais dentários.
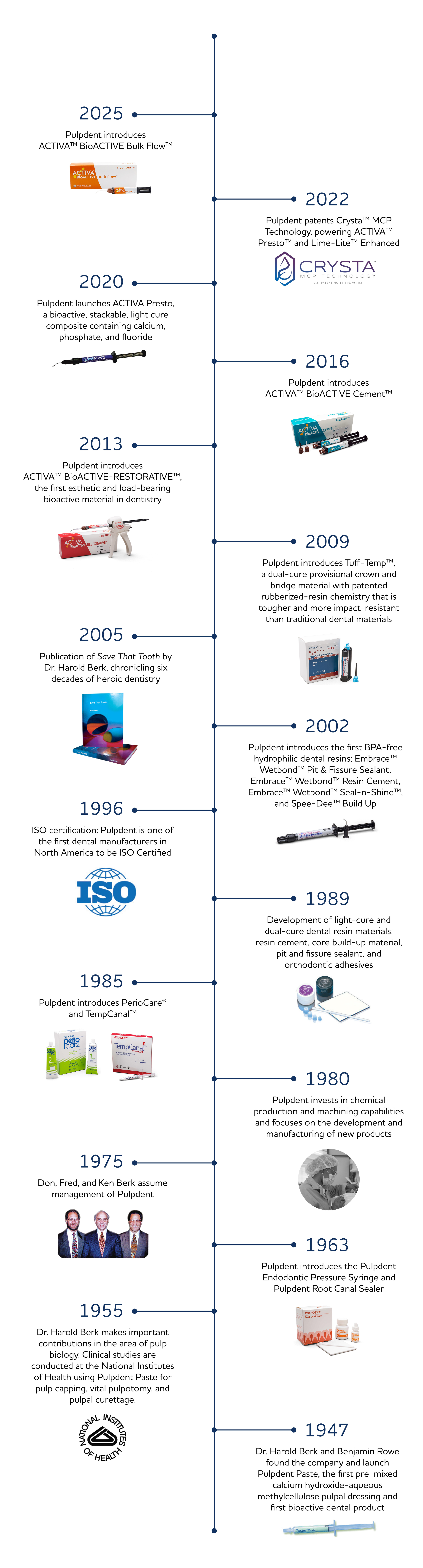
Linha de tempo da Pulpdent
Passa o cursor sobre cada data para saber mais
1947
Dr. Harold Berk and Benjamin Rowe found the company and launch Pulpdent Paste, the first pre-mixed calcium hydroxide-aqueous methylcellulose pulpal dressing and first bioactive dental product

1955
Dr. Harold Berk makes important contributions in the area of pulp biology. Clinical studies are conducted at the National Institutes of Health using Pulpdent Paste for pulp capping, vital pulpotomy, and pulpal curettage.

1963
Pulpdent introduces the Pulpdent Endodontic Pressure Syringe and Pulpdent Root Canal Sealer

1975
Don, Fred, and Ken Berk assume management of Pulpdent

1980
Pulpdent invests in chemical production and machining capabilities and focuses on the development and manufacturing of new products

1985
Pulpdent introduces PerioCare® and TempCanal™


1989
Development of light-cure and dual-cure dental resin materials: resin cement, core build-up material, pit and fissure sealant, and orthodontic adhesives

1996
ISO certification: Pulpdent is one of the first dental manufacturers in North America to be ISO Certified

2002
Pulpdent introduces the first BPA-free hydrophilic dental resins: Embrace™ Wetbond™ Pit & Fissure Sealant, Embrace™ Wetbond™ Resin Cement, Embrace™ Wetbond™ Seal-n-Shine™, and Spee-Dee™ Build Up

2005
Publication of Save That Tooth by Dr. Harold Berk, chronicling six decades of heroic dentistry

2009
Pulpdent introduces Tuff-Temp™, a dual-cure provisional crown and bridge material with patented rubberized-resin chemistry that is tougher and more impact-resistant than traditional dental materials

2013
Pulpdent introduces ACTIVA™ BioACTIVE-RESTORATIVE™, the first esthetic and load-bearing bioactive material in dentistry

2016
Pulpdent introduces ACTIVA™ BioACTIVE Cement™

2020
Pulpdent launches ACTIVA Presto, a bioactive, stackable, light cure composite containing calcium, phosphate, and fluoride

2022
Pulpdent introduces Pulpdent patents Crysta™ MCP Technology, powering ACTIVA™ Presto™ and Lime-Lite™ Enhanced

2025
Pulpdent introduces ACTIVA™ BioACTIVE Bulk Flow™
































Responsabilidade social
A Pulpdent sempre teve um interesse especial na saúde pública e na saúde oral das crianças. Cooperamos com a comunidade de saúde pública e apoiamos muitas iniciativas de saúde pública através do Programa de Parceria de Saúde Pública da Pulpdent. Para solicitar um donativo em géneros para uma iniciativa de saúde pública , clica aqui.